Difference between revisions of "Lead acid battery construction"
(safety section) |
(→Safety: secured) |
||
| Line 35: | Line 35: | ||
When charged, the cell plates form weakly attached lead compounds, some of which are shed over time to the bottom of the cell. Thus once charged, cells should only be disposed of either at suitable facilities, or in accordance with current legislative requirements. | When charged, the cell plates form weakly attached lead compounds, some of which are shed over time to the bottom of the cell. Thus once charged, cells should only be disposed of either at suitable facilities, or in accordance with current legislative requirements. | ||
| + | Topped containers can be used for construction. If open topped batteries are made, they must be enclosed in a secured container in use to avoid risk of spills and child access. | ||
==See Also== | ==See Also== | ||
Revision as of 05:35, 9 December 2008
Lead acid batteries are a simple technology, and have changed little since the 1800s. Battery banks for offgrid use are also expensive, making home made battery banks an attractive option.
Modern mass produced batteries feature moulded plates, lead compound pre-charge and antimony, but these are all optimisations rather than core features, and cells omitting these work entirely satisfactorily.
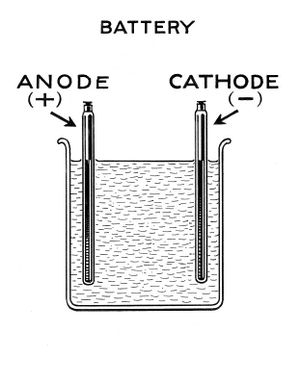
To make a lead acid cell requires only a glass or plastic container, lead roofing sheet, 4M sulphuric acid, deionised water, and some plastic to hold the lead plates in place. A hygrometer is used to achieve correct acid concentration.
Everything that goes into the cell must be thoroughly clean. Parts should be rinsed with deionised water before assembly.
Its recommended to make individual 2v cells, as suitable containers are easy to come by, the cells can easily be reconfigured, each cell can be checked or monitored, a bad cell is easily replaced, and handling weight is kept much lower.
The 2 electrodes are made of lead roofing sheet. Its cut to shape, washed & rinsed. Don't forget to leave a long tail on each electrode to enable wire connection to be made away from the acid bath. Capacity is in the region of 1Ah per one square inch of submerged anode and 1 square inch of submerged cathode.
A plastic bar or other suitable restraint holds the plates in place. This should be robust enough not to result in shorting during handling. Corrodable fixings (eg metal screws) must not be used. Plastic fixings are ok, such as plastic cable ties and plastic screws.
Wire connections to the lead plates should be made far enough away from the acid so as not to corrode from the inevitable acid spray. Connections and wire ends should be well coated in petroleum jelly - don't use other types of grease for electrical work. Such metal needs to be arranged so that any corrosion products won't run into the cell.
The battery bank should be enclosed in a container to keep debris out, and prevent accidental touching of acid or spillage. Batteries spray tiny amounts of acid in use, so should sit on something that can survive this.
Plates should be suspended at least 1/4" above the base of the cell container, and preferably 1/2" to allow accumulation of lead compounds without causing shorting. These compounds are gradually shed from the plates in normal operation.
Acid concentration is important but not critical, and should be in the range marked as healthy on the hygrometer. The acid weakens slightly during the first charge, and should be corrected after initial charge.
Full electrical capacity is only reached after several charges. For typical offgrid use this is not a problem, they can be put into service and will get themselves upto full capacity with no further atention.
Spare cell
Since the additional costs are minimal, a spare cell is handy to have. If one cell fails the spare can be wired in, avoiding long downtime. It should be stored dry.
Safety
Lead acid battery construction involves working with sulphuric acid, which has significant health hazards. If you don't know how to handle acids safely, try another project.
When charged, the cell plates form weakly attached lead compounds, some of which are shed over time to the bottom of the cell. Thus once charged, cells should only be disposed of either at suitable facilities, or in accordance with current legislative requirements.
Topped containers can be used for construction. If open topped batteries are made, they must be enclosed in a secured container in use to avoid risk of spills and child access.