Difference between revisions of "Lead acid battery construction"
(asstd, new sections) |
m (make spacing more consistent (i hope)) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
==Parts== | ==Parts== | ||
| − | To make a lead acid cell requires a glass or plastic container, lead [[roofing]] sheet | + | To make a lead acid cell requires a [[glass]] or [[plastic]] container, lead [[roofing]] sheet that's unused but no longer shiny, 4M [[Acid|sulphuric acid]], deionised water, petroleum jelly (eg vaseline) and some plastic to hold the lead plates in place. A hygrometer is used to achieve correct acid concentration. |
==Design features explained== | ==Design features explained== | ||
===Making life easy=== | ===Making life easy=== | ||
| − | Modern commercial [[Lead acid battery|batteries]] feature multiple moulded plates with separators, lead compound pre-charge and antimony, but these are | + | Modern commercial [[Lead acid battery|batteries]] feature multiple moulded plates with separators, lead compound pre-charge and antimony, but these are optimisations rather than core features, and cells omitting these are much quicker to make, and work entirely satisfactorily. Simplifying the construction makes this a practical project. |
| Line 16: | Line 16: | ||
Starting batteries, as used in cars, use interleaved lead plates to maximise cranking current. For off-grid use a much more durable plate layout is to use just 2 plates, widely separated. With this approach there is no need to connect multiple plates together or use separators. | Starting batteries, as used in cars, use interleaved lead plates to maximise cranking current. For off-grid use a much more durable plate layout is to use just 2 plates, widely separated. With this approach there is no need to connect multiple plates together or use separators. | ||
| − | The 2 electrodes are made of [[Sheet Materials|lead roofing sheet]]. Lead sheet can be rolled or folded to make an electrode with enough area. Just a little space should be left between the folds to allow ions to flow freely to all parts of the plates in use. | + | The 2 electrodes are made of oxidised [[Sheet Materials|lead roofing sheet]]. Lead sheet can be rolled or folded to make an electrode with enough area. Just a little space should be left between the folds to allow ions to flow freely to all parts of the plates in use. |
Spacing the 2 plates by 2" helps create a robust long lived cell. Even large amounts of plate distortion doesn't cause any problem. Folding the plates in such a way that each plate wraps around itself improves durability a little further by providing additional restraint against plate distortion. Eg: | Spacing the 2 plates by 2" helps create a robust long lived cell. Even large amounts of plate distortion doesn't cause any problem. Folding the plates in such a way that each plate wraps around itself improves durability a little further by providing additional restraint against plate distortion. Eg: | ||
| Line 36: | Line 36: | ||
===Electrical charging=== | ===Electrical charging=== | ||
| − | Plain lead plates only reach full electrical capacity after several charge cycles. These batteries however don't use plain lead, roofing lead | + | Plain lead plates only reach full electrical capacity after several charge cycles. These batteries however don't use plain lead, roofing lead acquires an oxidised surface, and experience so far is that they worked after one charge. The gradual increase in capacity with repeated charging is most easily addressed in an offgrid installation by simply putting them into service, where they can gradually get themselves upto full capacity without further attention. |
The battery may need a first charge using a charger that delivers above the rated battery voltage. This can be a mains charger, or where mains isn't present a lower than 12v battery may be connected to a 12v system for its first charge. Never parallel cells or [[battery|batteries]] when doing this! Don't leave cells on overvoltage for long if the charging circuit can deliver a high current, due to risk of them boiling and producing choking acid fumes. | The battery may need a first charge using a charger that delivers above the rated battery voltage. This can be a mains charger, or where mains isn't present a lower than 12v battery may be connected to a 12v system for its first charge. Never parallel cells or [[battery|batteries]] when doing this! Don't leave cells on overvoltage for long if the charging circuit can deliver a high current, due to risk of them boiling and producing choking acid fumes. | ||
| Line 45: | Line 45: | ||
==Construction== | ==Construction== | ||
| − | Everything that goes into the cell must be thoroughly clean. All internal parts need to be rinsed with deionised water before assembly. The lead roofing sheet is washed with | + | Everything that goes into the cell must be thoroughly [[clean]]. All internal parts need to be rinsed with deionised water before assembly. The lead roofing sheet is washed with tap[[water]], then rinsed off with deionised water before use. |
Its preferable to make individual 2v cells rather than 12v devices in one container, as | Its preferable to make individual 2v cells rather than 12v devices in one container, as | ||
| − | * suitable containers are easier to come by | + | |
| − | * construction is easier | + | *suitable containers are easier to come by |
| − | * the battery can easily be reconfigured | + | *construction is easier |
| − | * each cell can be checked or monitored individually | + | *the battery can easily be reconfigured |
| − | * a bad cell is easily replaced | + | *each cell can be checked or monitored individually |
| − | * handling weight is kept much lower | + | *a bad cell is easily replaced |
| − | * handling is easier | + | *handling weight is kept much lower |
| − | * less weight means much reduced risk of injury | + | *handling is easier |
| + | *less weight means much reduced risk of injury | ||
| Line 61: | Line 62: | ||
The 2 electrodes are made of [[Sheet Materials|lead roofing sheet]]. Its cut to shape, washed & rinsed. Don't forget to leave a long tail on each electrode to enable wire connection to be made away from the acid bath. 2 tails makes keeping it in place easier. Capacity is in the region of 1Ah per one square inch of submerged anode and 1 square inch of submerged cathode. | The 2 electrodes are made of [[Sheet Materials|lead roofing sheet]]. Its cut to shape, washed & rinsed. Don't forget to leave a long tail on each electrode to enable wire connection to be made away from the acid bath. 2 tails makes keeping it in place easier. Capacity is in the region of 1Ah per one square inch of submerged anode and 1 square inch of submerged cathode. | ||
| − | Plastic bars or other suitable restraints hold the plates in place. This must be robust enough not to result in shorting during handling. Corrodable fixings (eg metal [[screws]]) must not be used. Plastic fixings are ok, such as plastic cable ties and plastic screws. All fixings exposed to acid need to be cleaned & rinsed with deionised water before use. | + | Plastic bars or other suitable restraints hold the plates in place. This must be robust enough not to result in shorting during handling. Corrodable fixings (eg metal [[screws]]) must not be used. Plastic fixings are ok, such as plastic cable ties and [[plastic]] screws. All fixings exposed to [[acid]] need to be cleaned & rinsed with deionised water before use. |
Plates should be suspended at least 1/4" above the base of the cell container to allow accumulation of lead compounds without causing shorting. These compounds are gradually shed from the plates in normal operation. | Plates should be suspended at least 1/4" above the base of the cell container to allow accumulation of lead compounds without causing shorting. These compounds are gradually shed from the plates in normal operation. | ||
| Line 67: | Line 68: | ||
===Connections=== | ===Connections=== | ||
| − | [[cable|Wire]] connections to the lead plates should be made far enough away from the acid so as not to corrode from the inevitable acid spray. Connections and wire ends should be completely coated in petroleum jelly - don't use other types of grease for electrical work. The connection needs to be arranged so that any copper corrosion products won't run into the cell. A simple way to achieve this is to cut a tail on the end of each plate, and bring the tail out of the top of the cell container and down the outside. | + | [[cable|Wire]] connections to the lead plates should be made far enough away from the acid so as not to corrode from the inevitable [[acid]] spray. Connections and wire ends should be completely coated in petroleum jelly - don't use other types of grease for [[electrical]] work. The connection needs to be arranged so that any copper corrosion products won't run into the cell. A simple way to achieve this is to cut a tail on the end of each plate, and bring the tail out of the top of the cell container and down the outside. |
____________ | ____________ | ||
| | | | | | ||
| Line 92: | Line 93: | ||
===Containers=== | ===Containers=== | ||
| − | Battery containers need to not react with [[Acid|sulphuric acid]], and not conduct electrically. Plastic and glass are good options. They must be strong enough to survive all handling without any risk of breakage. Containers need tops/lids to much reduce the gradual acid spray that occurs in use. Tops also make construction a bit easier and reduce the chance of spillage. | + | [[Battery]] containers need to not react with [[Acid|sulphuric acid]], and not conduct electrically. Plastic and glass are good options. They must be strong enough to survive all handling without any risk of breakage. Containers need tops/lids to much reduce the gradual acid spray that occurs in use. Tops also make construction a bit easier and reduce the chance of spillage. |
In use, the entire battery bank should be enclosed in a durable secured container to keep debris out, prevent accidental touching of acid (eg by children) and avoid spillage. Batteries spray tiny amounts of acid in use, so should sit on something that can survive or neutralise this. The usual options are a lined wooden container or concrete. | In use, the entire battery bank should be enclosed in a durable secured container to keep debris out, prevent accidental touching of acid (eg by children) and avoid spillage. Batteries spray tiny amounts of acid in use, so should sit on something that can survive or neutralise this. The usual options are a lined wooden container or concrete. | ||
| Line 103: | Line 104: | ||
Lead acid battery construction involves working with [[Acid|sulphuric acid]], which has significant health hazards. Sulphuric acid eats flesh & eyeballs if given the chance. If you don't know how to handle strong [[acid]]s safely, this project isn't for you. | Lead acid battery construction involves working with [[Acid|sulphuric acid]], which has significant health hazards. Sulphuric acid eats flesh & eyeballs if given the chance. If you don't know how to handle strong [[acid]]s safely, this project isn't for you. | ||
| − | When charged, the cell plates form weakly attached lead compounds, some of which are shed over time to the bottom of the cell. | + | When charged, the cell plates form weakly attached lead compounds, some of which are shed over time to the bottom of the cell. Lead oxide is toxic. Once charged, cells should only be disposed of either at suitable facilities, or in accordance with current legislative requirements. |
Batteries should be kept in a secured container in use to avoid risk of spills and child access. | Batteries should be kept in a secured container in use to avoid risk of spills and child access. | ||
| Line 113: | Line 114: | ||
Tupperware style plastic containers are available from kitchen goods retailers, pound shops etc. | Tupperware style plastic containers are available from kitchen goods retailers, pound shops etc. | ||
| − | Deionised water is available from chemists and car accessory dealers. It can be harvested from [[dehumidifiers]] and a/c units, but | + | Deionised water is available from chemists and car accessory dealers. It can be harvested from [[dehumidifiers]] and a/c units, but they should be cleaned out first, and the water checked for conductivity, as contamination is very possible. |
| − | Hygrometers are available from car accessory | + | Hygrometers are available from car accessory shops. |
===Acid=== | ===Acid=== | ||
[[Acid|4M sulphuric acid]] has been bought from lab reagent suppliers and lead acid battery manufacturers. | [[Acid|4M sulphuric acid]] has been bought from lab reagent suppliers and lead acid battery manufacturers. | ||
| − | Reagent grade vitriol can be used, but increases the risk involved. Drain cleaning sulphuric acid is far too impure, and purifying it | + | Reagent grade vitriol can be used, but increases the risk involved. Drain cleaning sulphuric acid is far too impure, and purifying it impractical. |
Harvesting from scrap lead acid batteries is a gamble, as any slight ionic contamination discharges the cells, making them useless. If you're determined to do it, make a test cell using a couple of little bits of lead, charge it in the prospective acid, and test its self discharge time. If it can hold charge for a month, the acid's good. (Correct the acid strength before testing.) | Harvesting from scrap lead acid batteries is a gamble, as any slight ionic contamination discharges the cells, making them useless. If you're determined to do it, make a test cell using a couple of little bits of lead, charge it in the prospective acid, and test its self discharge time. If it can hold charge for a month, the acid's good. (Correct the acid strength before testing.) | ||
| Line 129: | Line 130: | ||
Plastic separators reduce the risk of a cell short due to impact or insufficient electrode support. They also make it practical to put the electrodes closer together, and thus increase the capacity per size. | Plastic separators reduce the risk of a cell short due to impact or insufficient electrode support. They also make it practical to put the electrodes closer together, and thus increase the capacity per size. | ||
| − | Bear in mind that homemade cells usually use pure lead electrodes with little support, whereas commercial batteries use lead alloyed with antimony to stiffen it, and electrodes fitted into slots in the casing for support. So homemade cells without separators require wide plate spacing and | + | Bear in mind that homemade cells usually use pure lead electrodes with little support, whereas commercial batteries use lead alloyed with antimony to stiffen it, and electrodes fitted into slots in the casing for support. So homemade cells without separators require wide plate spacing and gentle handling. |
| + | ===Phosphoric acid=== | ||
| + | Phosphoric acid isn't normally added to lead acid cells. Its addition increases capacity and longevity, but only if kept within a narrow range of concentration. If you're willing to monitor the electrolyte periodically and correct the acid concentration, it can be of use for a large battery bank, or to upgrade existing cells. Its not worthwhile for unmaintained batteries, which drift out of its narrow operating concentration range. | ||
| + | |||
| + | ===Further information=== | ||
| + | [https://archive.org/details/storageofelectri031249mbp/page/n8 Early publication] about lead acid battery cells by Gaston Plante written between 1859 to 5879. | ||
| + | [https://archive.org/details/voltaicaccumula00berlgoog/page/n4 Overview of different cell constructions] from 1889 by Reynier Émile. Chapter III and IV dedicated to solid plate designs. | ||
| − | + | [https://worldwide.espacenet.com/patent/search/family/032614094/publication/GB189800932A?q=GB189800932A British Patent GB189800932]from 1898 describing improved plate conditioning. | |
| − | |||
| + | [https://archive.org/details/storagebatteries0000vina Detailed Book] with in-depth information about the chemistry and theory of various battery types. First published 1924, written by George Wood Vinal. | ||
==See Also== | ==See Also== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| + | *[[Lead acid battery]] | ||
| + | *[[Special:Allpages|Wiki Contents]] | ||
| + | *[[Special:Categories|Wiki Subject Categories]] | ||
[[Category:Electrical]] | [[Category:Electrical]] | ||
[[Category:Batteries]] | [[Category:Batteries]] | ||
[[Category:Low Voltage]] | [[Category:Low Voltage]] | ||
Latest revision as of 23:27, 2 January 2020
Lead acid batteries are a simple technology, and have changed little since the 1800s. Battery banks for offgrid use are expensive, making home made battery banks an attractive option.
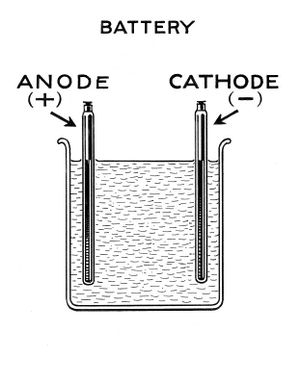
Parts
To make a lead acid cell requires a glass or plastic container, lead roofing sheet that's unused but no longer shiny, 4M sulphuric acid, deionised water, petroleum jelly (eg vaseline) and some plastic to hold the lead plates in place. A hygrometer is used to achieve correct acid concentration.
Design features explained
Making life easy
Modern commercial batteries feature multiple moulded plates with separators, lead compound pre-charge and antimony, but these are optimisations rather than core features, and cells omitting these are much quicker to make, and work entirely satisfactorily. Simplifying the construction makes this a practical project.
Plate design
Starting batteries, as used in cars, use interleaved lead plates to maximise cranking current. For off-grid use a much more durable plate layout is to use just 2 plates, widely separated. With this approach there is no need to connect multiple plates together or use separators.
The 2 electrodes are made of oxidised lead roofing sheet. Lead sheet can be rolled or folded to make an electrode with enough area. Just a little space should be left between the folds to allow ions to flow freely to all parts of the plates in use.
Spacing the 2 plates by 2" helps create a robust long lived cell. Even large amounts of plate distortion doesn't cause any problem. Folding the plates in such a way that each plate wraps around itself improves durability a little further by providing additional restraint against plate distortion. Eg:
_____ _____ ___ | _ | | _ | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |_| | | | | | | | | OR | _ | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |___| | | |___| |___|
The plates need to be suspended off the base of the container, because they gradually shed lead compounds which will short the cell otherwise. The gap between plates and bottom should be at least 0.25", and a generous 0.5" makes for a cell proof against even heavy shedding.
Electrical charging
Plain lead plates only reach full electrical capacity after several charge cycles. These batteries however don't use plain lead, roofing lead acquires an oxidised surface, and experience so far is that they worked after one charge. The gradual increase in capacity with repeated charging is most easily addressed in an offgrid installation by simply putting them into service, where they can gradually get themselves upto full capacity without further attention.
The battery may need a first charge using a charger that delivers above the rated battery voltage. This can be a mains charger, or where mains isn't present a lower than 12v battery may be connected to a 12v system for its first charge. Never parallel cells or batteries when doing this! Don't leave cells on overvoltage for long if the charging circuit can deliver a high current, due to risk of them boiling and producing choking acid fumes.
Spare cell
Since the additional costs are minimal, a spare cell is handy to have. If one cell fails the spare can be wired in, avoiding long downtime. It should be stored dry to acheive indefinite storage life. Lead acid cells don't survive long if stored wet & not reguarly charged.
Construction
Everything that goes into the cell must be thoroughly clean. All internal parts need to be rinsed with deionised water before assembly. The lead roofing sheet is washed with tapwater, then rinsed off with deionised water before use.
Its preferable to make individual 2v cells rather than 12v devices in one container, as
- suitable containers are easier to come by
- construction is easier
- the battery can easily be reconfigured
- each cell can be checked or monitored individually
- a bad cell is easily replaced
- handling weight is kept much lower
- handling is easier
- less weight means much reduced risk of injury
Plates
The 2 electrodes are made of lead roofing sheet. Its cut to shape, washed & rinsed. Don't forget to leave a long tail on each electrode to enable wire connection to be made away from the acid bath. 2 tails makes keeping it in place easier. Capacity is in the region of 1Ah per one square inch of submerged anode and 1 square inch of submerged cathode.
Plastic bars or other suitable restraints hold the plates in place. This must be robust enough not to result in shorting during handling. Corrodable fixings (eg metal screws) must not be used. Plastic fixings are ok, such as plastic cable ties and plastic screws. All fixings exposed to acid need to be cleaned & rinsed with deionised water before use.
Plates should be suspended at least 1/4" above the base of the cell container to allow accumulation of lead compounds without causing shorting. These compounds are gradually shed from the plates in normal operation.
Connections
Wire connections to the lead plates should be made far enough away from the acid so as not to corrode from the inevitable acid spray. Connections and wire ends should be completely coated in petroleum jelly - don't use other types of grease for electrical work. The connection needs to be arranged so that any copper corrosion products won't run into the cell. A simple way to achieve this is to cut a tail on the end of each plate, and bring the tail out of the top of the cell container and down the outside.
____________ | | | | | | | | | | | |__________|_| Cut the plate
_
| |
| |
| |
__________| |
| |_|
| |
| |
| |
|__________|
Fold the tail upward, then down over the side.
Containers
Battery containers need to not react with sulphuric acid, and not conduct electrically. Plastic and glass are good options. They must be strong enough to survive all handling without any risk of breakage. Containers need tops/lids to much reduce the gradual acid spray that occurs in use. Tops also make construction a bit easier and reduce the chance of spillage.
In use, the entire battery bank should be enclosed in a durable secured container to keep debris out, prevent accidental touching of acid (eg by children) and avoid spillage. Batteries spray tiny amounts of acid in use, so should sit on something that can survive or neutralise this. The usual options are a lined wooden container or concrete.
Acid
Acid concentration is important, but not critical, and should be in the range marked as healthy on the hygrometer. The acid weakens slightly during the first charge, and acid is easier to dilute than strengthen, so the cells can be filled with acid toward the stronger end of the range initially. Acid concentration should be corrected after initial charge.
Safety
Lead acid battery construction involves working with sulphuric acid, which has significant health hazards. Sulphuric acid eats flesh & eyeballs if given the chance. If you don't know how to handle strong acids safely, this project isn't for you.
When charged, the cell plates form weakly attached lead compounds, some of which are shed over time to the bottom of the cell. Lead oxide is toxic. Once charged, cells should only be disposed of either at suitable facilities, or in accordance with current legislative requirements.
Batteries should be kept in a secured container in use to avoid risk of spills and child access.
Supplies
Lead sheet is available at any builder's merchants or DIY shed.
Tupperware style plastic containers are available from kitchen goods retailers, pound shops etc.
Deionised water is available from chemists and car accessory dealers. It can be harvested from dehumidifiers and a/c units, but they should be cleaned out first, and the water checked for conductivity, as contamination is very possible.
Hygrometers are available from car accessory shops.
Acid
4M sulphuric acid has been bought from lab reagent suppliers and lead acid battery manufacturers.
Reagent grade vitriol can be used, but increases the risk involved. Drain cleaning sulphuric acid is far too impure, and purifying it impractical.
Harvesting from scrap lead acid batteries is a gamble, as any slight ionic contamination discharges the cells, making them useless. If you're determined to do it, make a test cell using a couple of little bits of lead, charge it in the prospective acid, and test its self discharge time. If it can hold charge for a month, the acid's good. (Correct the acid strength before testing.)
Extras
Separators
Plastic separators reduce the risk of a cell short due to impact or insufficient electrode support. They also make it practical to put the electrodes closer together, and thus increase the capacity per size.
Bear in mind that homemade cells usually use pure lead electrodes with little support, whereas commercial batteries use lead alloyed with antimony to stiffen it, and electrodes fitted into slots in the casing for support. So homemade cells without separators require wide plate spacing and gentle handling.
Phosphoric acid
Phosphoric acid isn't normally added to lead acid cells. Its addition increases capacity and longevity, but only if kept within a narrow range of concentration. If you're willing to monitor the electrolyte periodically and correct the acid concentration, it can be of use for a large battery bank, or to upgrade existing cells. Its not worthwhile for unmaintained batteries, which drift out of its narrow operating concentration range.
Further information
Early publication about lead acid battery cells by Gaston Plante written between 1859 to 5879.
Overview of different cell constructions from 1889 by Reynier Émile. Chapter III and IV dedicated to solid plate designs.
British Patent GB189800932from 1898 describing improved plate conditioning.
Detailed Book with in-depth information about the chemistry and theory of various battery types. First published 1924, written by George Wood Vinal.